HiBiT-Based Detection of PCSK9 Secretion: A Powerful Tool for Protein Trafficking Studies
Simon Moe, Christopher Eggers, and Megan Luedeman
Promega Corporation
Publication date: February 2025
Introduction
HiBiT is an 11-amino acid peptide tag developed for highly sensitive bioluminescent detection and quantification of proteins. Its small size is ideal for tracking protein expression and localization due to minimal disruption of the tagged protein’s function and structure. HiBiT exhibits high-affinity binding to its complementary partner, LgBiT, forming an active luminescent enzyme. When a bioluminescent substrate is introduced, this interaction generates a strong, quantifiable luminescent signal directly proportional to the concentration of HiBiT-tagged proteins, facilitating precise and real-time protein analysis. Compared to traditional immunoassays such as ELISA, which captures protein levels at a fixed endpoint, or Western blotting, which requires protein separation and transfer, HiBiT allows real-time tracking of protein dynamics with higher sensitivity and reduced background noise.
HiBiT is a powerful tool for studying extracellular protein activity, including the secretion of proteins, due to the impermeability of LgBiT. In the Nano-Glo® HiBiT Extracellular Detection System, LgBiT is added to live-cell media, where it binds exclusively to HiBiT-tagged proteins outside of the cell, allowing selective quantification of the extracellular protein population. This system enables the study of various extracellular protein activities, including receptor internalization and recycling, protein trafficking to the cell surface, and protein secretion from the cell.
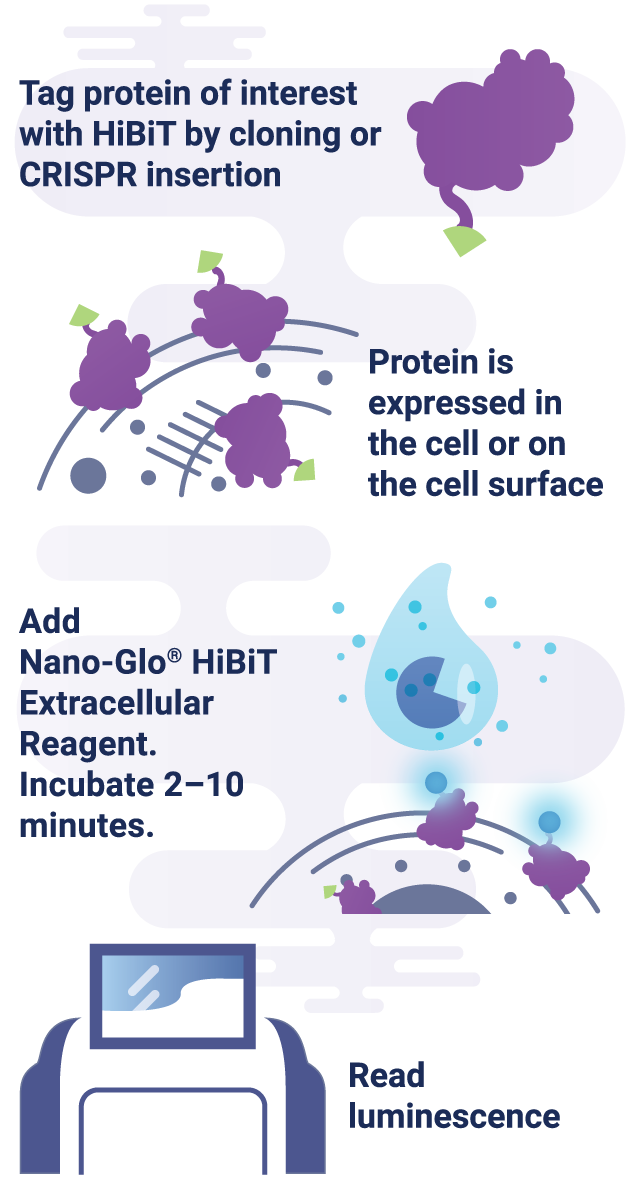
Case Study: Using HiBiT to Study PCSK9 Secretion
Promega scientists sought to demonstrate the power of HiBiT technology for studying the secretion of extracellular proteins. As a model system, they investigated the processing and secretion of proprotein convertase subtilisin/kexin type 9 (PCSK9). PCSK9 is closely associated with lipid regulation: its overexpression is linked to hyperlipidemia, while reduced secretion or gene knockout leads to hypolipidemia (Abifadel et al., 2003; Cohen et al., 2006). Given this strong connection, PCSK9 has emerged as a compelling therapeutic target, inspiring the development of two FDA-approved antibody therapies—Praluent (Sanofi/Regeneron) and Repatha (Amgen).
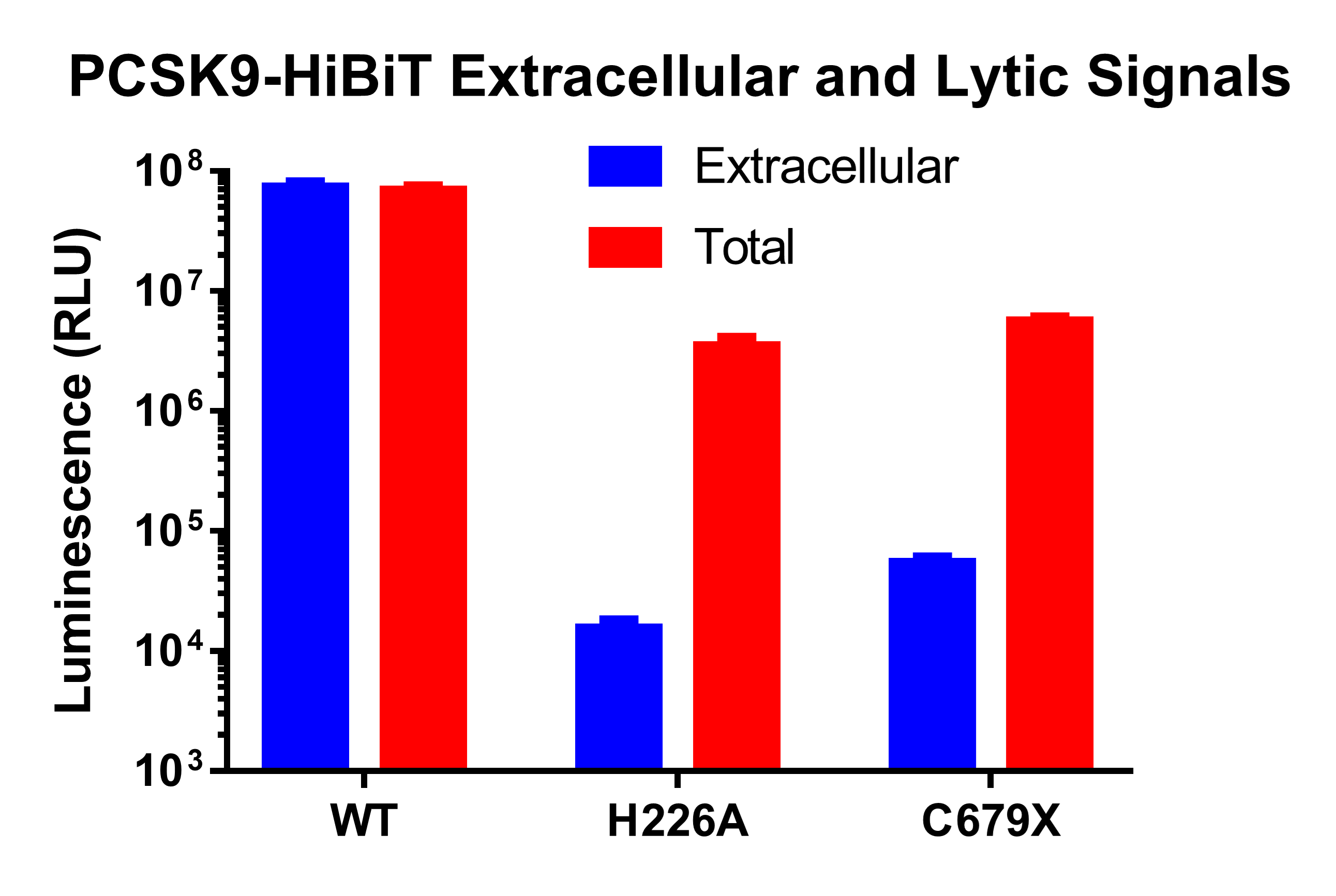
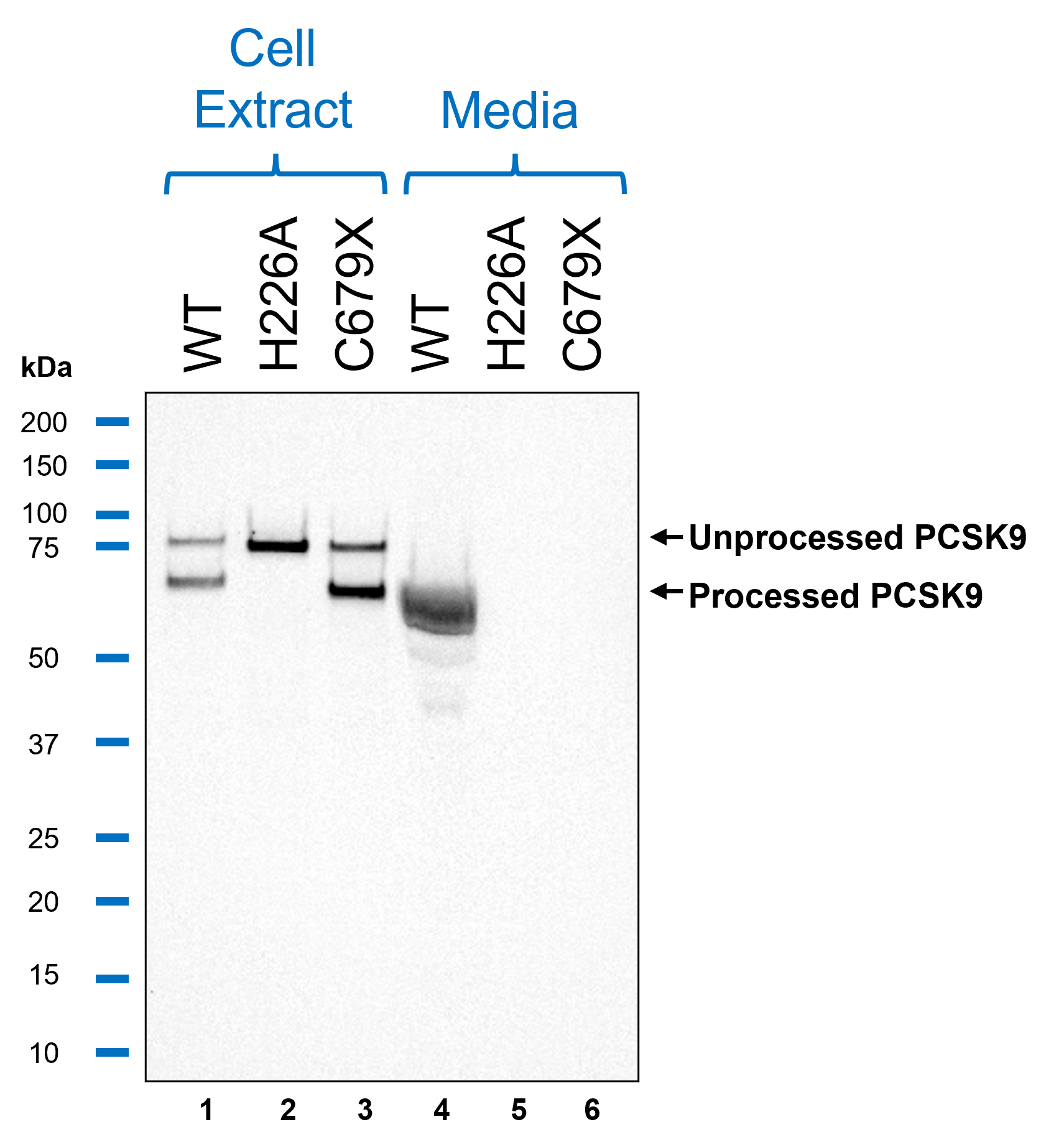
Prior research has established the secretion behavior of PCSK9. Before secretion, PCSK9 undergoes an autoproteolytic process within the endoplasmic reticulum relying on a protease domain. Modifications in the genome of PCSK9, including those that impact this autoproteolytic process, result in changes in secretion. One of these is the H226A mutation, which blocks auto-proteolytic processing, causing retention in the endoplasmic reticulum (Benjannet et al., 2004). A second is the C679X mutation, which causes PCSK9 misfolding and leads to retention in the endoplasmic reticulum without inhibiting proteolysis (Zhao et al., 2006).
HiBiT-Based PCSK9 Secretion Assay
HiBiT Blotting
Measuring PCSK9 Secretion Over Time
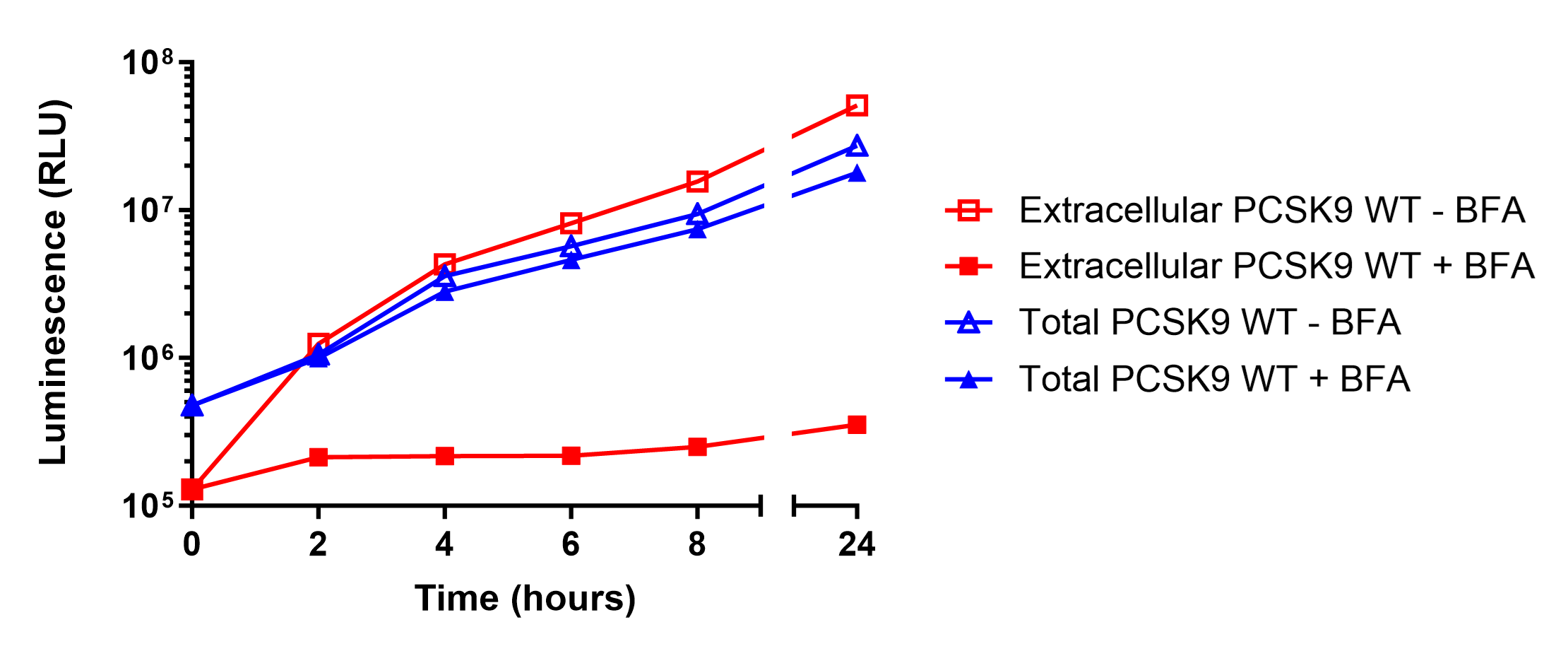
Conclusions
This study demonstrates how HiBiT can be used to track protein expression, processing, and secretion with high sensitivity and precision. Using HiBiT-based assays, we confirmed that wild-type PCSK9 is efficiently secreted, while the H226A and C679X mutations cause intracellular retention by distinct mechanisms. The H226A mutation prevents autoproteolytic processing, leading to ER retention, whereas the C679X mutation disrupts proper protein folding, also resulting in intracellular accumulation. These results validate HiBiT as a powerful tool for detecting changes in protein trafficking using both plate-based luminescent detection and HiBiT Blotting, providing a low-background, highly quantitative alternative to traditional methods.
Beyond PCSK9, HiBiT assays are broadly applicable to the study of extracellular protein dynamics, including the monitoring of GPCR trafficking, and for facilitating the measurement in protein trafficking to and from the plasma membrane. The ability to introduce HiBiT via CRISPR-mediated genome editing further expands its utility, enabling the study of proteins at endogenous expression levels with appropriate regulation. Promega offers a wide variety of CRISPR Ready-to-Use reporter cell lines premade with HiBiT tags. Additionally, real-time kinetic secretion measurements can be performed by adding LgBiT Protein and a stable substrate directly to the culture media, allowing continuous luminescent tracking of secretion events over time.
With these expanded applications, HiBiT serves as a powerful and versatile tool for investigating protein trafficking, secretion mechanisms, and therapeutic protein production across both basic and applied research.
Citations
- Benjannet, S., et al (2004). NARC-1/PCSK9 and its natural mutants: Zymogen cleavage and effects on the low density lipoprotein (LDL) receptor and LDL cholesterol. Journal of Biological Chemistry, 279(47), 48865–48875. https://doi.org/10.1074/jbc.M409699200
- Horton, J. D., et al (2007). Molecular biology of PCSK9: its role in LDL metabolism. In Trends in Biochemical Sciences (Vol. 32, Issue 2, pp. 71–77). https://doi.org/10.1016/j.tibs.2006.12.008
- Zhao, Z., et al (2006). ARTICLE Molecular Characterization of Loss-of-Function Mutations in PCSK9 and Identification of a Compound Heterozygote. In The American Journal of Human Genetics (Vol. 79). www.ajhg.org
Learn More
Related Resources
Technical Article: HiBiT, A Tiny Tag Expanding Endogenous Protein Detection
If you’re obsessed with a protein and immunoassays are driving you crazy, the HiBiT tag might make your life a little easier.
Targeted Protein Degradation Services
Accelerate the discovery and development of degrader molecules with our comprehensive screening and profiling services.